Surface tension denoted with the Greek variable gamma is defined as the ratio of the surface force F to the length d along which the force acts. Equating the fundamental quantities into the equation we get MLT-2 L-1.
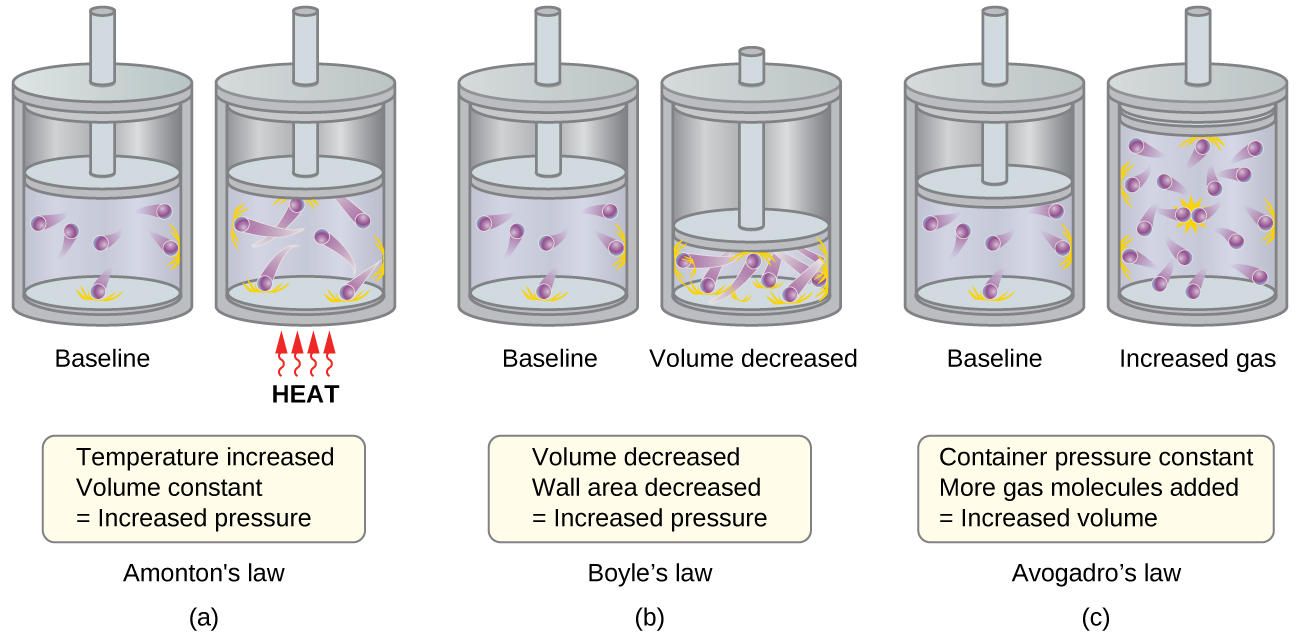
Gas Behavior Kinetic Molecular Theory And Temperature M5q5 Uw Madison Chemistry 103 104 Resource Book
Surfacetension moleculartheory class12physics hscboard SURFACE TENSION FULL CHAPTER PLAYLIST -httpswwwyo.
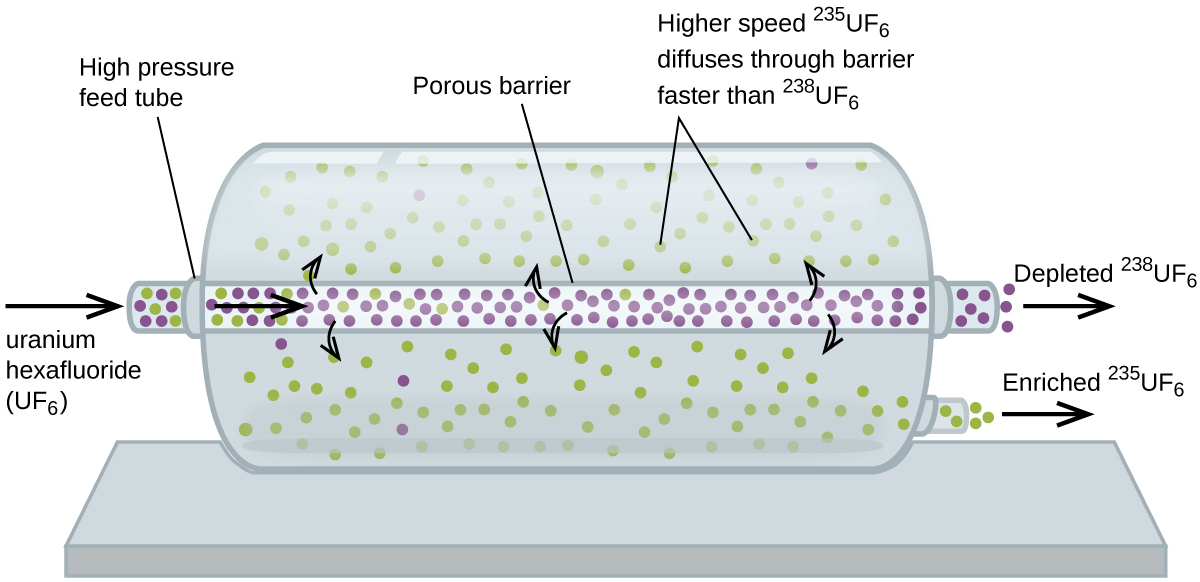
. Solving further we get MT-2. The force required to pull the ring from the surface of the. It combines the concepts of cohesion and adhesion.
Statistical-Mechanical Treatment of the Surface Tension. Stresses in the Transition Layer. Rx and Ry are radii of curvature in each of the axes that are parallel to the surface.
Ask Question Asked 3 years 10 months ago. Molecular Structure of Water. Hence the dimensional formula of surface tension is MT-2.
Critique of the Assumption of a Mathematical Plane of Density Discontinuity. The tendency of water surface to contract and resemble an elastic membrane is known as surface tension of water. In physics surface tension is an effect within the surface layer of a liquid that causes the layer to behave as an elastic sheet.
Scalable illustration of capillary action for large and. Δp is the pressure difference known as the Laplace pressure. The Helmholtz free energy the quantity minimized in the van der Waals approach is.
Approximate Theories of Surface Tension. Gamma F d. Use the editor to format your answer Paint Question 23 Calculate the molality of the following solution.
Surface tension diagrams. Whats the cause for that. Water drops tend to form spheres.
The energy associated with the surface is surface tension and can be expressed in units of dynes per centimeter dyn cm-1. The Water H 2 O molecule has a triangular geometry with O-H bond distance of 00965nm and the H-O-H bond angle is 1045. Δ p γ 1 R x 1 R y displaystyle Delta pgamma left frac 1 R_ x frac 1 R_ yright where.
Molecular theory of surface tension. Surface tension of liquid iron gallium lithium and lead was calculated by the molecular dy-namics method using embedded atom model EAM potential. 37g of glucose C6H1205 in 64g of water Use the editor to format your answer.
Molecular theory of surface tension part 2. Surfaces were formed by divi-sion of the molecular dynamics model in basic cube into two parts in the external field. Surface tension is defined as the force per unit length acting perpendicular on an imaginary line drawn on the liquid surface tending to pull the surface apart along the line.
Surface tension is a property of a liquid that allows them to resist external forces. A force that tends to pull adjacent parts of a liquids surface together thereby decreasing surface area to the smallest possible size. PS is the free surface of the liquid and QR is the inner layer parallel to PS at distance equal to the range of molecular force.
Surface tension is caused by a strong attraction between the molecules cohesion that cause them to link together and remain uniform even when placed on differing surfaces adhesion. Modified 1 year 1 month ago. As a result of.
Oxygen atom is more electronegative than the hydrogen atoms so it attracts. γ is surface tension. In this state it has less energy per unit of surface relative to volume.
Other examples of surface tension include water striders dew drops raindrops on a. The surface tension of liquids depends on the composition of the vapour phase. The ring is place in the surface of the liquid allowing the liquid to adhere to the ring.
A molecular theory of surface tension is developed for a liquidgas interface of a one component system. If F is the force acting on the length l of the line AB then surface tension is given by TFl. Surface tension molecular theory.
The directed contracting force which attracts the molecules at the surface of a liquid towards the interior of the liquid is surface tension. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Calculation of the Surface Energy.
As we know surface tension is given by the formula Surface tension FL. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance. Relation between the Surface Energy and the Heat of Vaporization.
Dunoys ring is a common method used to measure surface tension. I know that the surface molecules experience a net force downward so there is dense layer of water molecules in the. Thermodynamics of Surface Tension.
Use the editor to format your answer 1 Point Question 3 Describe the 4 standards that gases need to have in order to fit the kinetic molecular theory of gases. Although the water as a whole is electrically neutral it behaves as an electrical dipole. Free body diagram of DuNoys ring.
Here the water molecules in the droplet are held together by surface tension while they are in contact with the desk. Viewed 244 times 3 begingroup How does the surface of water behave like a stretched membrane. The force is perpendicular to the line and tangential to the liquid surface.
Along the surface the particles are pulled toward the rest of the liquid as shown in the picture to the right. Physics Assignment Help Explain the molecular theory of surface tension i On the average particles are separated by a distance of the order of 10-10 m and exert a force of attraction of the order of 10-11 N on each other. The surface tension of a liquid is mainly a force that mainly acts to reduce the surface area of a liquid.
The attraction of the surface of a liquid to the surface of a solid is a property closely related to. Now consider three molecules A B and C in a liquid in a vessel such that molecule A is well inside the liquid molecule B within the surface film and molecule C is on the surface of the liquid as shown in the figure. When the molecules possess.
Ii The force of attachment among the molecules is because of electrical interaction among. We know that F ma substituting the value in the equation we get maL. 28 405 1958 have pointed out that the difference of the work done in bringing a molecule from the interior to the water surface for malonic acid and that for succinic acid is much smaller than the increment to this work for each additional CH 2 group of long chain hydro-carbons with one end group attracted by water.
It is the effect that allows insects such as the water strider to walk on water and causes capillary action for example.

Surface Tension And Adsorption Studies By Drop Profile Analysis Tensiometry Kairaliyeva 2017 Journal Of Surfactants And Detergents Wiley Online Library

Gas Behavior Kinetic Molecular Theory And Temperature M5q5 Uw Madison Chemistry 103 104 Resource Book
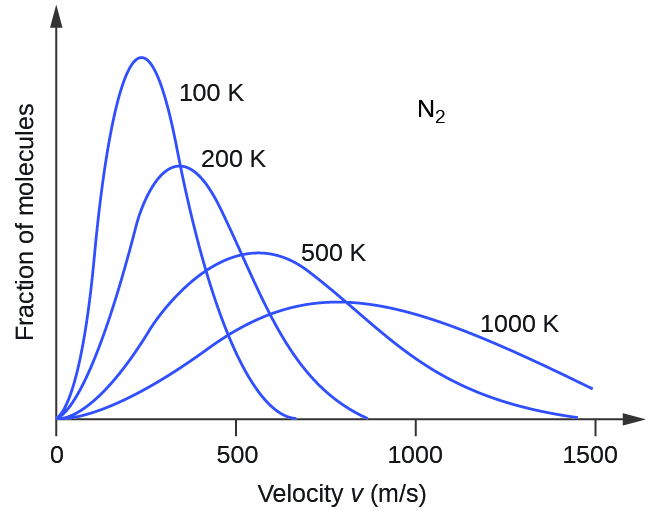
Gas Behavior Kinetic Molecular Theory And Temperature M5q5 Uw Madison Chemistry 103 104 Resource Book
0 Comments